quali-g™
Features of Quali-G™ capsules
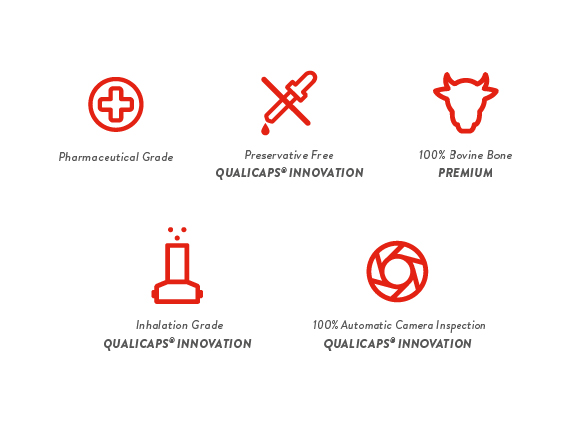
Quali-G™ capsules can be used at all stages in the development process, from pre-clinical toxicological testing through all phases of clinical trials, and finally to product launch and commercialization, as they do not undergo any changes in physical and chemical performance when empty throughout their shelf life.The GMP-compliant manufacturing process is carried out following strict pharmaceutical criteria and certified according to ISO 9001 and ISO 14001. Drug Master Files for the US and Canada have been registered. Quali-G™ capsules are continuously monitored by Quality Control experts in the production process, and are also inspected using an automatic camera system to detect and remove defective units.
Available in a wide range of color and sizes used in human medicine, ranging from 5 up to 00, Quali-G™ capsules can be further customized through printing (axial, radial and laser options available), which also functions as an effective anti-counterfeit measure.
An inhalation-grade option is available with improved microbial and weight specifications (Commercial information Capsules):
- Outstanding quality
- Favorable powder release from capsule shell
- Improved microbiological purity, particularly relevant as the drug material is delivered directly into the lungs
- Tighter weight specifications can be provided
- Compatible with a range of excipients that improve bioavailability of poorly soluble actives
- Leak-proof, even with liquid formulations when applying the patented band-sealing technology
- Tamper-evident, thanks to the proprietary band-sealing technology
- Provides an excellent barrier to oxygen penetration
- Allows for semi-solid matrix formulation fills at temperatures up to 70°C