QUALI-V® EXTRA DRY
Quali-V® Extra Dry, a cellulose capsule with an extremely low moisture content that enables the development, production and delivery of moisture-sensitive pharmaceutical drugs.
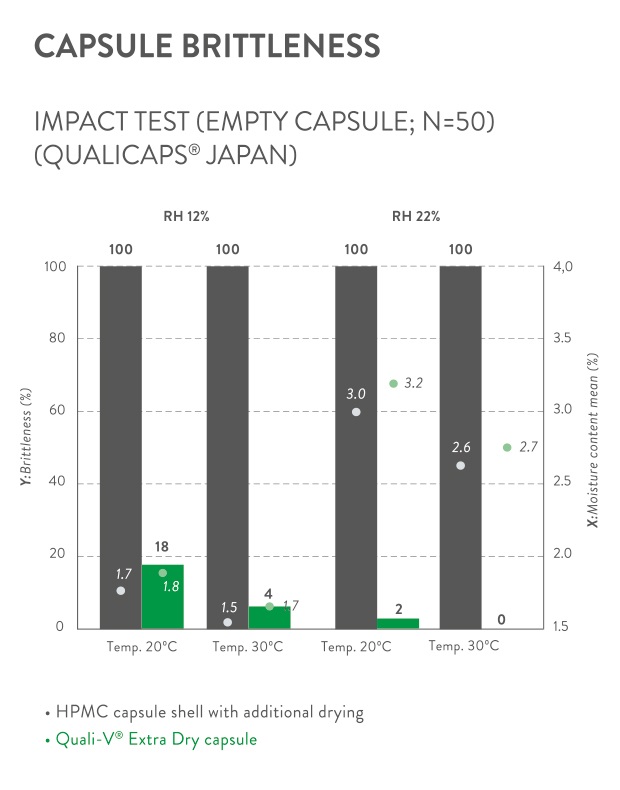
The stability, activity, and expiry date of several active pharmaceutical ingredients (APIs) under certain environmental conditions depend on the moisture content to which they are exposed. Also, specific hygroscopic compounds (e.g. polyethylene glycols, acid glycerol esters, acid triglycerides) widely used as excipients thanks to their outstanding solubility or absorption attributes, cannot be used inside gelatin or standard cellulose capsules without altering their properties.
FEATURES OF QUALI-V® EXTRA DRY
Quali-V® Extra Dry, with a unique composition of being 100% plant-based and preservative-free, is specifically designed for oral pharmaceutical applications when a minimum moisture content is required.Just as our standard Quali-V® capsules, Quali-V® Extra Dry capsules comply with the USP dissolution test requirements and are chemically inert (do not undergo cross-linking reactions).
An inhalation-grade version is available with improved mechanical properties and the required microbial pharmacopoeia specifications.
For further information this and other specific capsule products, please contact our Scientific Business Development experts.